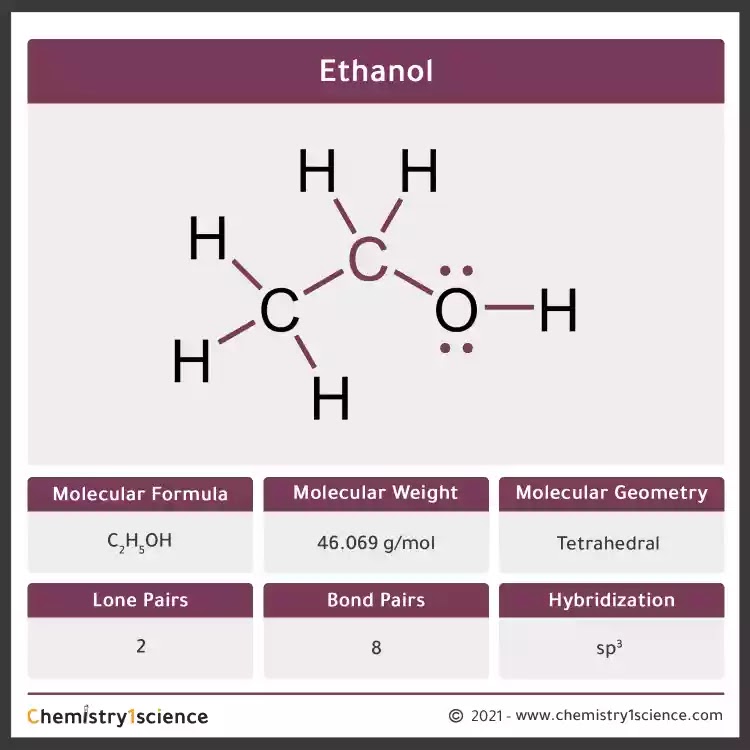
Ethanol Molecular Geometry Hybridization Molecular Weight
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Is Ethanol (C2H5OH) Polar or Nonpolar? Techiescientist
In the lewis structure of ethanol, all bonds between atoms are single bonds. One hydrogen atom has joint with oxygen atom and that oxygen atom is joint with one carbon atom. There are two lone pairs in the valence shell of oxygen atom. Steps of drawing lewis structure of CH 3 CH 2 OH

Ethanol Formula Composition, Uses, Structure, Density
Molecular weight: 46.0684 IUPAC Standard InChI: InChI=1S/C2H6O/c1-2-3/h3H,2H2,1H3 IUPAC Standard InChIKey: LFQSCWFLJHTTHZ-UHFFFAOYSA-N CAS Registry Number: 64-17-5 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript . Isotopologues: [2H6]ethanol

Ethanol Formula Formula, Composition, Uses, Structure Embibe
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
A Look At The Ethanol Lies
Lewis Dot Structure of CH3CH2OH (Ethanol) kentchemistry.com 25.1K subscribers Subscribe Subscribed 80K views 12 years ago Every Video I quickly take you through how to draw the Lewis Structure.

Schema De Lewis De Ethanol
Subscribed 9.6K views 2 years ago An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles..more.more An explanation of the.
/GettyImages-136810090-56a133b25f9b58b7d0bcfd93.jpg)
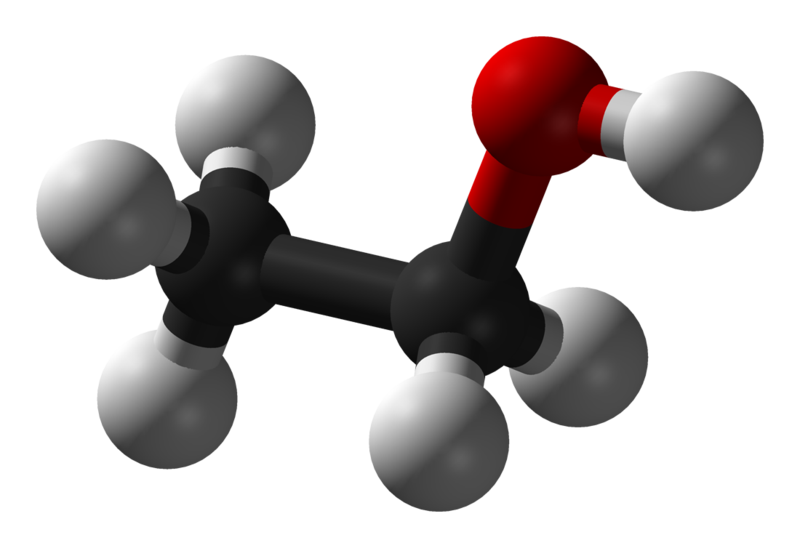
Ethanol Molecular Formula and Empirical Formula
Ethanol Lewis structure November 2, 2023 by Deep The information on this page is fact-checked. Ethanol Lewis structure CH 3 CH 2 OH or C 2 H 5 OH or C 2 H 6 O (ethanol) has two carbon atoms, six hydrogen atoms, and one oxygen atom. In the ethanol Lewis structure, there are five C — H bonds, one C — C bond, one C — O bond, and one O — H bond.
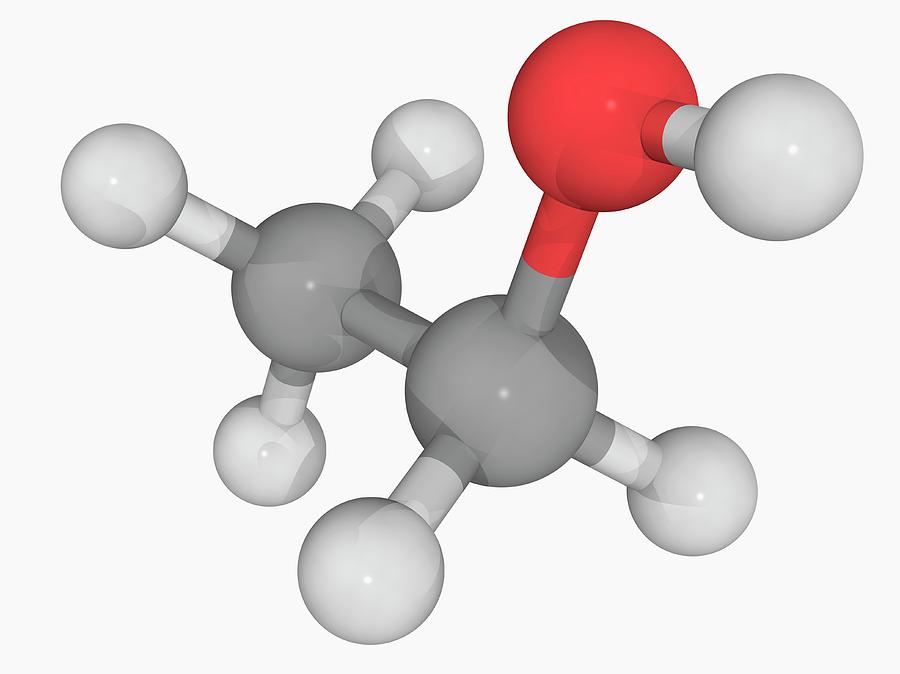
Ethanol Molecule Photograph by Laguna Design/science Photo Library Pixels
Lewis structure of C2H5OH (or Ethanol) contains five C-H bonds, one O-H bond and one C-O bond. The two Carbon atoms (C) are at the center and it is surrounded by Hydrogen atoms (H) and one OH group. The oxygen atom has 2 lone pairs. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try.

Structural chemical formula and model of ethanol Vector Image
Steps of drawing C2H5OH lewis structure Step 1: Find the total valence electrons in C2H5OH molecule. In order to find the total valence electrons in a C2H5OH molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
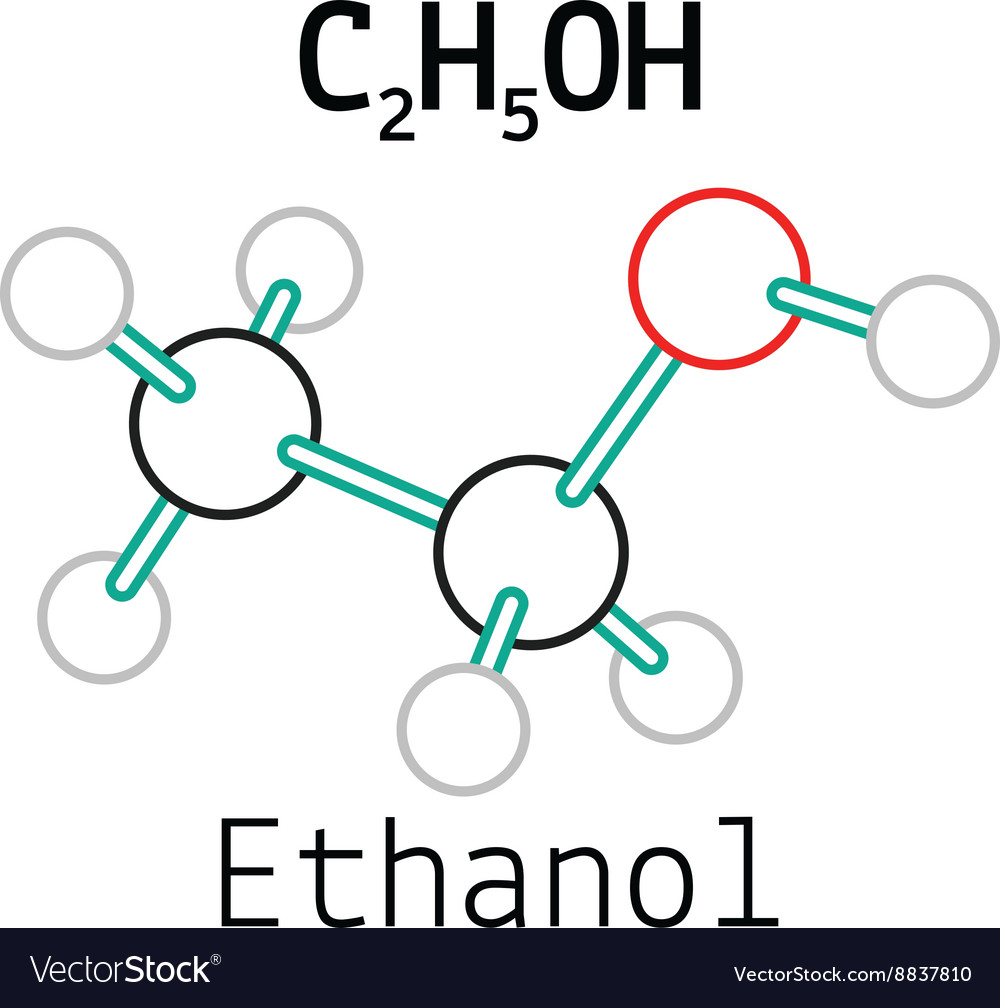
C2h5oh ethanol molecule Royalty Free Vector Image
A step-by-step explanation of how to draw the C2H5OH Lewis Dot Structure (Ethanol (Ethyl alcohol)).For the C2H5OH structure use the periodic table to find th.
Lewis Structure For Ethanol
How to Draw a Lewis Structure for Ethanol? C2H6OLewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Subscribe.

Éthanal Définition et Explications
10.1 Structure and Classification of Alcohols. Page ID. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. It examines in some detail their simple physical properties such as solubility and boiling points. Alcohols are compounds in which one or more hydrogen atoms in an alkane have been.

Lewis Structure For Ethanol
Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. This is a 2-D representation and it helps us to understand more about the properties of the compound. Let's move step-by-step and see how the Lewis Structure of C2H5OH can be made. Step 1: Finding the valence electrons for each atom.

Ethanol Formula Formula, Composition, Uses, Structure Embibe
The Lewis structure for ethyne, a linear molecule, is: The IUPAC nomenclature for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. For example, CH 3 CH 2 C ≡ CH CH 3 CH 2 C ≡ CH is called 1-butyne.

"Ethanol (Alcohol) Structural Formula" Sticker for Sale by
The Lewis structure is a simple yet powerful tool for understanding the bonding and arrangement of atoms in a molecule. In ethanol, we have a combination of carbon (C), hydrogen (H), and oxygen (O) atoms. To construct the Lewis structure, we start by counting the valence electrons. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has.
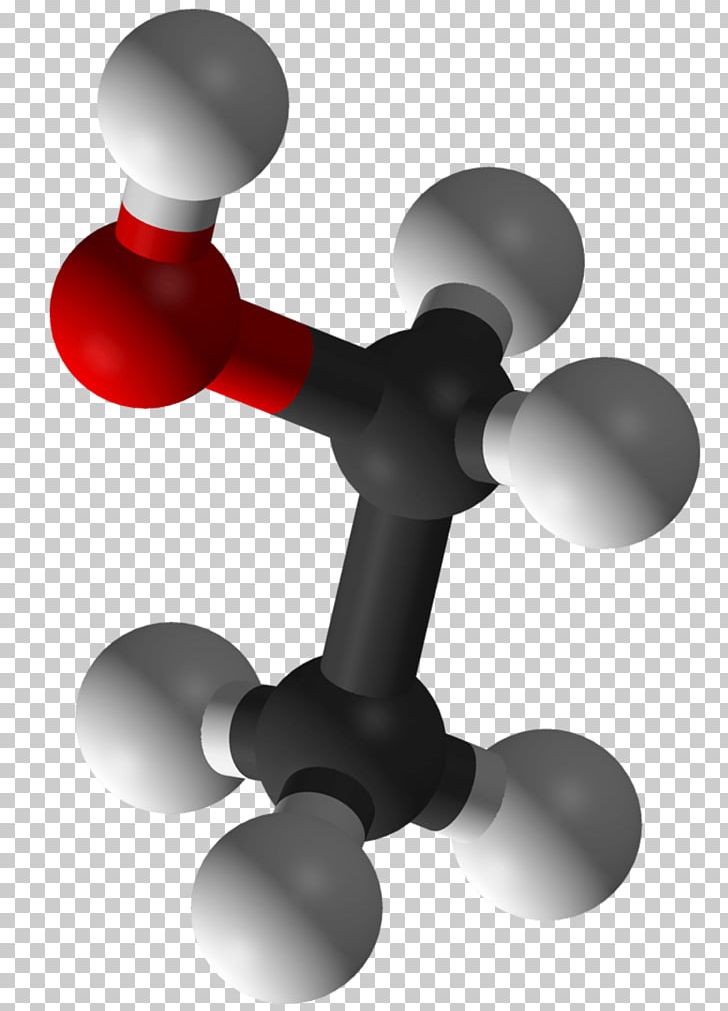
Ethanol Molecule Alcohol Universe Chemistry PNG, Clipart, Alcohol
C2H5OH or Ethanol is an organic chemical compound, which can also be represented as CH3-CH2-OH. Ethanol is a colourless liquid with a distinct odour and has a pungent taste. It has flammable properties; and gives a blue colour flame when burnt.